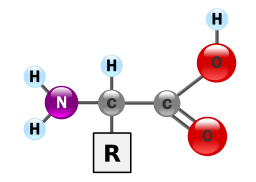
 Proteins provide much of the structural and functional capacity of cells. Proteins are composed of monomers called amino acids. Amino Acids are hydrocarbons that have an amino group (-NH2) and an acidic carboxyl group (-COOH).The R group represents a hydrocarbon chain with a modification that alters the properties of the amino acid. 20 universal amino acids are used to construct proteins. The variation in functional groups along the amino acid chain gives rise to the functional diversity of proteins.
Proteins provide much of the structural and functional capacity of cells. Proteins are composed of monomers called amino acids. Amino Acids are hydrocarbons that have an amino group (-NH2) and an acidic carboxyl group (-COOH).The R group represents a hydrocarbon chain with a modification that alters the properties of the amino acid. 20 universal amino acids are used to construct proteins. The variation in functional groups along the amino acid chain gives rise to the functional diversity of proteins.


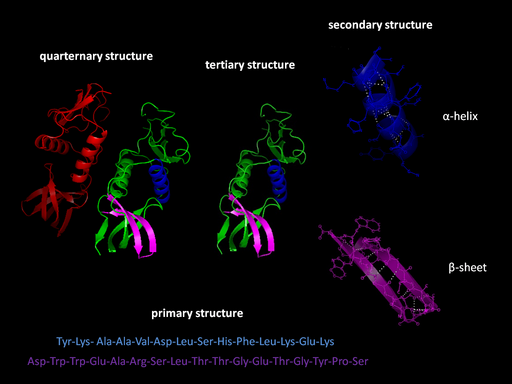
Levels of structure
- Primary Structure (1°): The sequence of amino acids read from the Amino or N-terminal end of the molecule to the Carboxyl or C-terminal end
- Secondary Structure (2°): local three-dimensional structures that form from interactions of amino acids, like hydrogen bonding
- Alpha Helix – coils occurring from the H-bonds between N-H and C=O groups along the backbone of the protein
- Beta Sheets – laterally connected strands or sheets of amino acids occurring from the H-bonds between N-H and C=O groups along the backbone of the protein
- Tertiary structure (3°): overall 3-D structure of the peptide chain
- Quaternary structure(4°): multimeric protein structure from assembling multiple peptide subunits