Understanding Membranes
The cell membrane is the barrier that separates the cytoplasm from the internal cytoplasm with the external world. The cell membrane consists primarily of phospholipids in a bilayer. Phospholipids are amphipathic with a polar head (phosphate group) and a hydrophobic tail (2 hydrocarbon chains). Due to the chemical properties of the heads being attracted to water and the tails having a desire to avoid water, phospholipids self assemble into micelles. Cell membranes form from a phospholipid bilayer where the lipid tails interact with each other and the phosphate heads face the external water environment or the internal cytoplasm of the cell.
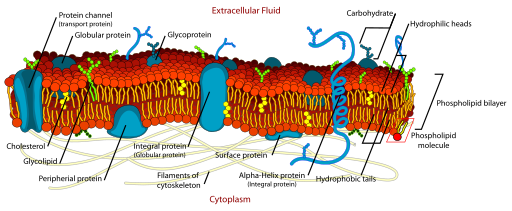
 The cell membrane does not solely consist of phospholipids but also have proteins and cholesterol inserted into the bilayer. As the image of the bilayer above indicates, the molecules are constantly moving and flow in a lateral motion. Cholesterol modulates the fluidity of this motion. Proteins associated with the membrane may sit on either side (peripheral proteins) of the membrane or pass through both layers of the membrane (transmembrane proteins). The model that describes the components of the cellular membrane is referred to as the Fluid Mosaic Model. This model states that the cell membrane is a mosaic of 1)Phospholipids 2)Proteins 3) cholesterol that move about in a side to side motion.
The cell membrane does not solely consist of phospholipids but also have proteins and cholesterol inserted into the bilayer. As the image of the bilayer above indicates, the molecules are constantly moving and flow in a lateral motion. Cholesterol modulates the fluidity of this motion. Proteins associated with the membrane may sit on either side (peripheral proteins) of the membrane or pass through both layers of the membrane (transmembrane proteins). The model that describes the components of the cellular membrane is referred to as the Fluid Mosaic Model. This model states that the cell membrane is a mosaic of 1)Phospholipids 2)Proteins 3) cholesterol that move about in a side to side motion.

Diffusion

- Temperature Effects on Diffusion
- Molecular Mass Effects on Diffusion
Osmosis
Osmosis is a special case of diffusion. Instead of observing the net change in solute, osmosis follows the net movement of solvent across a semipermeable membrane. Since a semi-permeable membrane permits specific things to pass through, some solutes are partitioned.

A cell lacking a cell wall is affected greatly by the tonicity of the environment. In a hypertonic solution where the concentration of dissolved solute is high, water will be drawn out of the cell. In a hypotonic solution where the concentration of dissolved solute is lower than the interior of the cell, the cell will be under great osmotic pressure from the environmental water moving in and can rupture.
Plants have rigid cell walls composed of cellulose. These cell walls permit for maintenance of cellular integrity when the external environment is hypotonic (less dissolved substances). In this situation, the water moves into the cell. Without the cell wall, the cell would burst open from the excessive water pressure entering the cell. This state of swelling is referred to as turgid, resulting from turgor pressure.

When the exterior environment is hypertonic , (greater amount of dissolved substances), the reverse condition occurs whereby the cellular fluid exiting the cell reduces the size of the cytoplasm. This condition is referred to as plasmolysis

