Properties of Light
Light is a type of energy that travels as a wave-particle.The wavelength of light is the distances between peaks in the waves as light travels. Wavelengths are measured in nanometers (nm) and different wavelengths of light represent differing colors. White light is a mixture of the visible light spectrum. Light of long wavelengths (infra-red) and very short wavelengths (ultra violet) are invisible to humans but can be observed by other organisms. As wavelength decreases, the energy of the light is increased.

Diffraction of light through a prism exposes the components wavelengths of light.
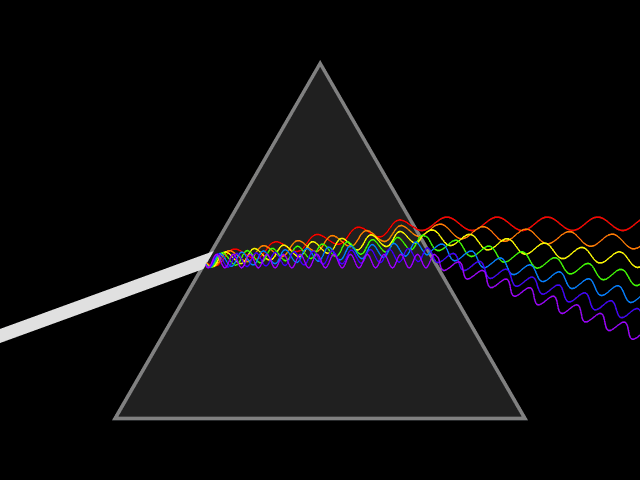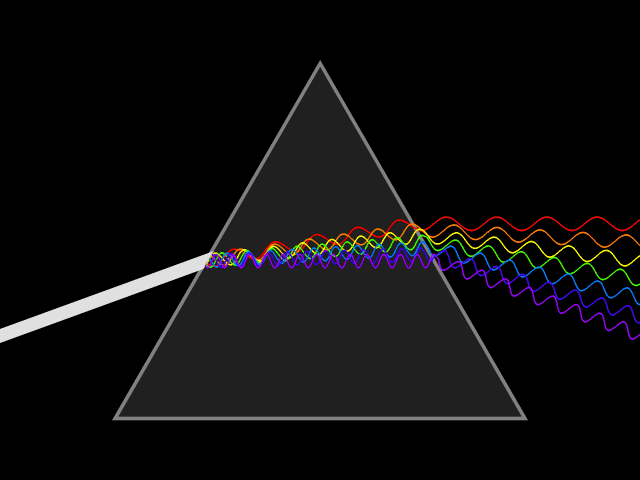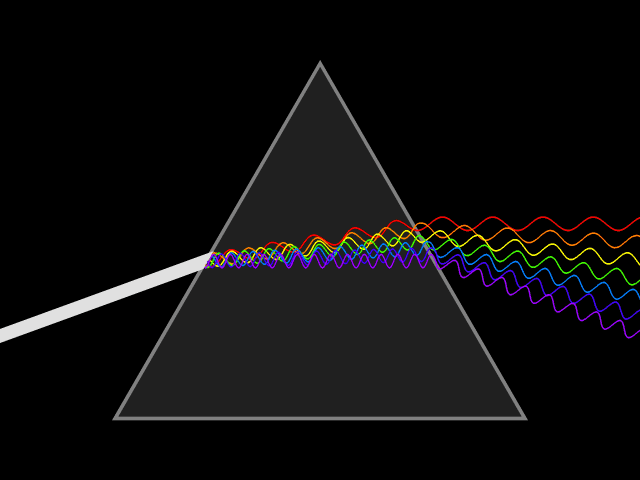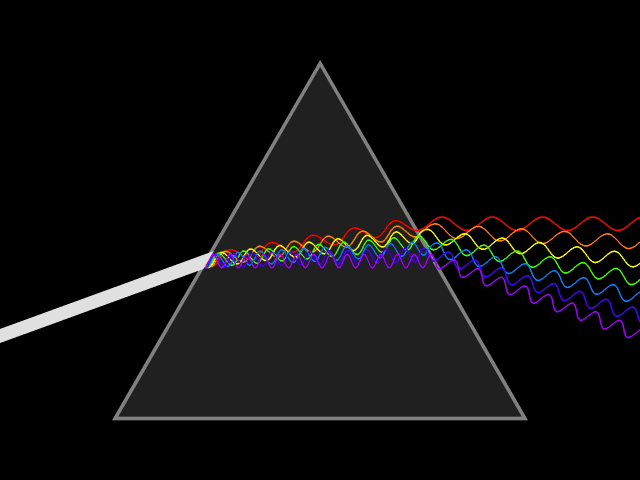
Spectrophotometry
Spectrophotometers (spectro-image/color ; photo-light ; meter-measure) are used for chemical analysis of solutions based on properties of absorption or transmission.
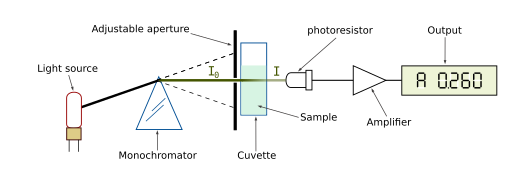

If light is being absorbed by chemicals in the solution, this results in a lower transmission. Absorbance is therefor inversely related to transmittance as expressed by the equation:
![]()
Follow the virtual demonstration at: http://www.virtual-labs.leeds.ac.uk/pres/spectrophotometry/ (CC-BY-NC-SA) for a more in-depth explanation of spectrophotometry.
Beer’s Law
Beer’s Law is a relationship between the concentration or amount of a dissolved substance in a solution that is reducing the amount of transmitted light due to the absorption of the radiant energy. Lambert’s Law states that the reduction of transmittance was related to the length of the path of light. As the light path increases through a substance, there is a reduction in transmittance. Collectively, these ideas are referred to as Beer-Lambert Law, but most observers will control the path length and simply refer to it as Beer’s Law.

